Reference: Lobbé, Q., Chavalarias, D., Delanoë, A., Ferrand, G., Cohen-Boulakia, S., Ravaud, P., Boutron, I., 2022. Toward an observatory of the evolution of clinical trials through phylomemy reconstruction: the COVID-19 vaccines example. Journal of Clinical Epidemiology. https://doi.org/10.1016/j.jclinepi.2022.05.004
Abstract
Objective. To visualize the evolution of all registered COVID-19 vaccines trials
Study Design and Setting. As part of the living mapping of the COVID-NMA initiative, we identify biweekly all COVID-19 vaccine trials and automatically extract data from the EU clinical trials registry, ClinicalTrials.gov, IRCT and the WHO International Clinical Trials Registry Platform. Data are curated and enriched by epidemiologists.
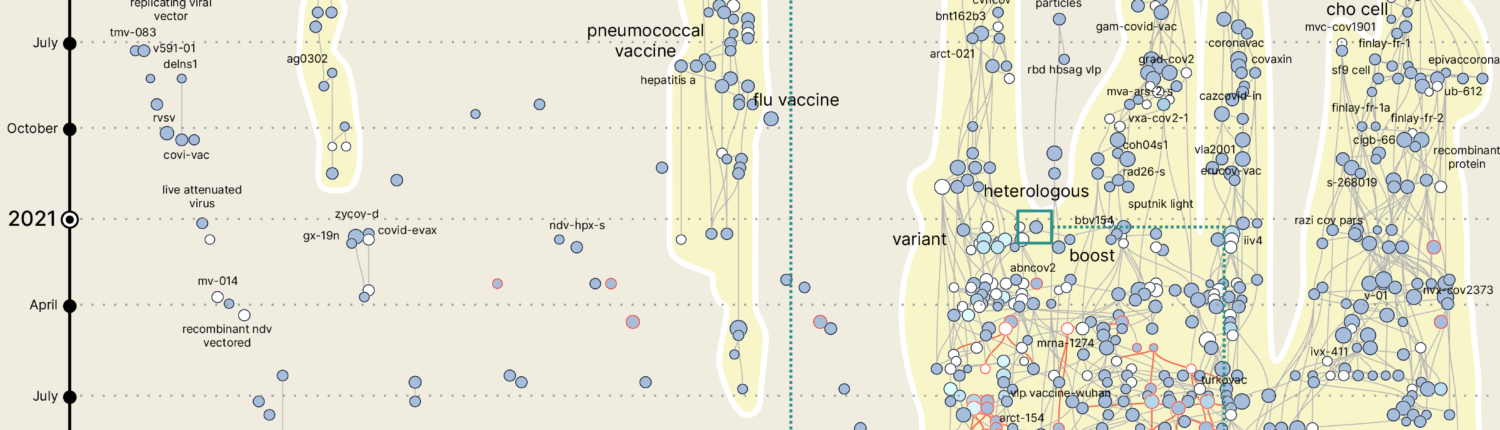
We have used the phylomemy reconstruction process to visualize the temporal evolution of COVID-19 vaccines trials descriptions. We have analyzed the textual contents of 1,794 trials descriptions (last search in October 2021) and explored their collective structure along with their semantic dynamics.
Results. The structures highlighted by the phylomemy reconstruction processes synthesize the complexity of the knowledge produced by the research community. The reconstructed phylomemy clearly retrieves the five major COVID-19 vaccine platforms in the form of complete branches. The branches interactions reflect the exploration of a new approach to vaccine implementation moving from homologous prime vaccination to heterologous prime vaccination. Phylomemies also clearly identifies shifts in research questions; from vaccine efficacy to booster efficacy.
Conclusion. this new method provides important insights for the global coordination between research teams especially in crisis situations such as the COVID-19 pandemic.
Authors
Lobbé, Q., Chavalarias, D., Delanoë, A., Ferrand, G., Cohen-Boulakia, S., Ravaud, P., Boutron,Pre-print of the paper
See the pre-print of the paper
View on Publisher’s website
- Full paper with SI on Springer (restricted access)
Interactive phylomemies
- Phylolemy with founding organization highlitht
- Phylomemy with countries highlight
- Phylomemy with phases highlight
- Phylomemy with publication status (Yes/No)
- Phylomemy with public tested (immunodepressed, newborn, pregnant, etc.)
Randomized/unrandomized Clinical trials